The Case for Point-of-Care 3D Printing
While hospitals aren’t ready to turn into on-site manufacturers, they are turning to service providers to ramp up point-of-care additive manufacturing for patient-specific medical devices.
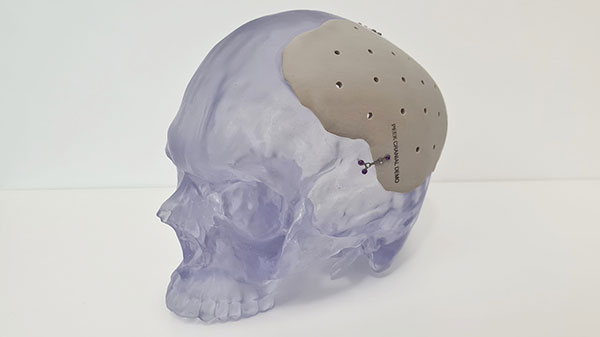
3D printed PEEK cranial implant successfully manufactured at point of care on 3D Systems’ EXT 220 Med (formerly Kumovis R1). Image courtesy of 3D Systems.
September 15, 2023
Materialise recently took the wraps off the latest edition to its Plymouth, MI, production facility: A 10,000-sq.-ft. space devoted to metal 3D printing of personalized medical devices complete with a robust printer fleet, post-processing equipment, clean room and a packaging and assembly setup. The new factory, designed to turn out patient-specific implants and surgical guides, was a couple of years in the making and cost millions of dollars—a reality most hospitals and medical centers aren’t ready to replicate on their own, despite increasing interest in point-of-care (POC) additive manufacturing (AM).
“It’s a fairly elaborate process that few institutions are ready to take on,” says Bryan Crutchfield, vice president and general manager, Materialise NA, linking rising U.S. market demand for AM-produced patient-specific devices to Materialise’s decision to bankroll a new metal AM factory. “For now, these large investments are made by traditional manufacturers that are set up for quality procedures and are used to working in the heavily regulated environment. Most hospitals aren’t there yet.”
In fact, only a select few hospitals—the Veterans Administration (VA) and the Mayo Clinic among them—have started experimenting with what it takes to build on-site, internally run manufacturing capabilities that are U.S. Food and Drug Administration (FDA) certified to produce patient-specific tools like surgical cutting guides and hip, knee and cranial implants at scale.
3D printing anatomical models used to help patients understand diagnoses and procedures are more widespread among the medical community, but once the use case calls for a 3D-printed instrument or medical device as part of a procedure or to be implanted in the human body, things become more complicated with far fewer hospitals ready to take the plunge.
Specifically, medical device manufacturing is a highly regulated industry, and while the FDA is still translating those requirements to POC AM, there are significant hurdles for hospitals lacking expertise in this area. Moreover, the FDA has clearly established a requirement for a manufacturing-grade quality management system (QMS) and processes—a competency lacking in most hospitals. POC AM also demands significant staffing investments in areas like engineering, 3D printing, quality management and regulatory expertise—which are outside the scope and budgets of all but the largest care centers.
“It takes a lot of money and time—it’s not for the faint of heart,” says Dr. Beth Ripley, acting director, Office of Healthcare Innovation and Learning at the Veterans Health Administration (VHA), adding that the VA is fully committed to exploring all options for POC AM.
“We deliberately set out to try all scenarios, all the way through medical device manufacturing, so we can understand the pros and cons of each and share that out,” she says.
Currently, the VA has gotten FDA 510(k) clearance for two patient-matched devices. One is the OroMaxilloFacial Advanced Surgical Planning System, a surgical guide device for reconstructive surgeries of the jaw and face, and the other, a device used to deliver customized radiation therapy for cancer treatments. Although these are major milestones, Ripley says the VA’s focus is now on simplification so it can expand the program to reach more device types and patients.
“We are now focused on processes and types of devices and technologies that we can package up, test and vet, and standardize so we can get POC AM into all our hospitals, not just a few,” Ripley says.
Challenges to POC AM
Regulatory compliance is perhaps the biggest challenge to hospitals standing up their own on-site POC AM capabilities. In December 2021, the FDA drafted a revised framework that outlines three manufacturing scenarios for 3D-printed devices at POC, yet there isn’t formal guidance on specific requirements or insight into how resulting products would be evaluated for risk. The three scenarios are:
- A healthcare facility deploys a specially designed medical device production system (MDPS), built by a traditional manufacturer and run by the healthcare facility, but where regulatory compliance lies with the manufacturer;
- A traditional manufacturer co-locates near or at a healthcare site and takes responsibility for operations and regulatory compliance;
- A healthcare facility assumes all traditional manufacturer roles and responsibilities.
More recently, the FDA released additional draft guidance, this time for patient-matched surgical guides for orthopedic implants, and is asking for input. Although both papers are critical steps toward bringing clarity to the risk-based regulatory environment surrounding POC AM, there is still enough ambiguity to slow widespread adoption.
“The challenge today is that we don’t have black-and-white guidance for the regulatory pathways a hospital must follow,” says Beatriz Gonzalez, global market manager for 3D printing at POC at Materialise. “It’s great that we are now talking about regulation—that means we’re seeing more value and broad adoption, but it’s still emerging.”
The need to build and implement a manufacturing-grade quality management system (QMS) to ensure AM-produced patient devices are accurate, safe and repeatable is another big hurdle for hospitals, even for pioneers like the VA and the Hospital for Special Surgery (HSS). A medical device manufacturing QMS is not something you can buy off the shelf as it must specifically fit the needs and processes of your organization, Ripley says, adding that the VA has an advantage as it already maintains trained manufacturing and quality management professionals on staff.

Anatomical model printed with the RICOH 3D for Healthcare medical manufacturing center aids in surgical collaboration. Image courtesy of Ricoh USA.
HSS, which produced implants on-site with traditional manufacturing methods for years, eventually got out of the business due to the complex regulatory and QMS challenges, notes Joseph Lipman, director of device development, biomechanics, at the hospital.
Today, the hospital has partnered with LimaCorporate S.p.A., which operates the fully FDA-certified ProMade POC Center at the hospital’s campus; the center provides HSS physicians and patients with the benefits of fast-turnaround POC AM for patient-specific guides and implants without the hospital and its staff shouldering the manufacturing, quality, regulatory and staffing burdens.
“We get the benefit of having a local partner, which improves communication and delivers access to technology we might not be aware of,” Lipman says. “It’s a nice way to go without having to take on the burden of regulatory requirements to maintain a 3D printing facility, which for metal printing is significant.”
In fact, the mechanics of metal 3D printing is not conducive to a clinical environment—the melting and postprocessing steps create sanitary and health risks and can be dangerous without the proper expertise and safeguards. Metal 3D printers are expensive and sizable, eating into already strained hospital budgets and resources, and the still limited number of cases at individual hospitals makes the economics a hard sell.
“The ecosystem that goes with metal 3D printing is daunting,” says Andy Christensen, a veteran of medical use cases for AM for decades and the president of Fingerprint Additive LLC, a medical AM consultancy. “You can’t run that kind of facility with a handful of people so the economics don’t make a lot of sense.”
Creating an on-site medical device manufacturing hub also demands new kinds of talent, including engineers trained in 3D printing technologies as well as the software used to segment patient imaging data into a format that can be 3D printed. There is also a need for clinical engineers who can work with surgeons to translate their surgical plan into a 3D-printable guide or custom device.
“You can find someone who knows 3D printing well and train them on the healthcare aspects or have someone who knows healthcare who can learn 3D printing, but you rarely see folks who have both sets of skills,” says Ben Johnson, vice president, portfolio and regulatory for 3D Systems.
Vendor Solutions Pave Way for POC AM
3D Systems has efforts underway to facilitate POC AM, including a consulting practice where experts can be contracted to serve on-site at hospitals or alternatively, and can train internal staff to prepare them for key roles, according to Johnson. In one such example, 3D Systems contributed to the VHA’s work to establish FDA-compliant manufacturing facilities in hospitals. 3D Systems’ acquisition of German medical 3D printer manufacturer Kumovis also expands its POC AM portfolio with regulatory approved systems for high-performance polymers such as polyether ether ketone (PEEK). The University Hospital of Salzburg successfully leveraged the EXT 220 Med (formerly Kumovis R1) platform to produce its first patient-specific 3D-printed PEEK cranial implant at its onsite printing lab in concert with the 3D Systems team, which helped with application development, qualification of printers and the regulatory process.
Stratasys has also taken steps to fortify its POC AM footprint. The company has a variety of FDA-cleared workflow solutions for creating accurate anatomical models for diagnostic use with the key players in this space, including Materialise, Synopsys and Axial3D, which offer FDA 510(k) cleared image segmentation software.
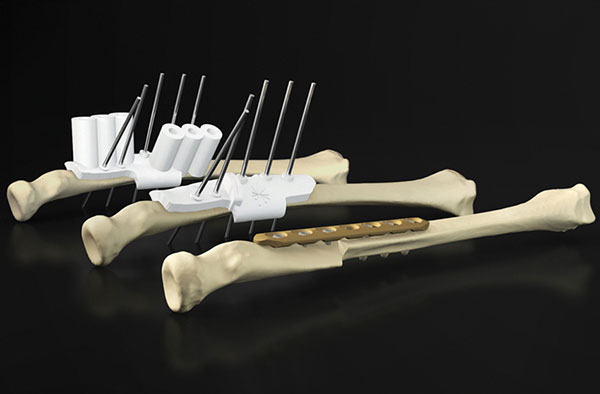
Patient-specific drilling guides are one popular use case for 3D printing at the point of care. Image courtesy of Materialise.
Stratasys has also made a $10 million investment in Axial3D, working with the firm to make patient-specific 3D printing solutions more accessible. A partnership with RICOH 3D for Healthcare is also central to its POC AM strategy, according to Scott Drikakis, global medical segment leader, Americas, at Stratasys. RICOH 3D for Healthcare is an integrated HIPAA-enabled 3D medical manufacturing center and workflow for developing and producing patient-specific 3D-printed anatomic models that are powered by Stratasys 3D printing technologies.
The RICOH 3D for Healthcare offering is available as an on-demand option, where anatomical models can be ordered and printed at the RICOH facility, or a RICOH-managed services team will oversee a 3D printing lab on-site, says Gary Turner, managing director AM, at RICOH USA.
The solution has received FDA 510(k) clearance for craniomaxillofacial and orthopedic anatomic modeling and more recently for 3D anatomic modeling of soft tissue for diagnostic use in cardiovascular, neurological, gastrointestinal, genitourinary and breast applications. Patient-specific surgical guides and implants are potentially on the horizon as the industry and regulatory climate matures, he says.
Though expectations and excitement for POC AM are high, the complexities can be daunting and the use cases will vary depending on the hospital, its patient profile, and how much time and resources they’re willing to invest.
“Every hospital has to decide what they want to be when they grow up as it relates to 3D printing and outline what the requirements are from there,” Drikakis says.
More 3D Systems Coverage
More Materialise Coverage
More Stratasys Coverage
Subscribe to our FREE magazine, FREE email newsletters or both!
About the Author
Beth Stackpole is a contributing editor to Digital Engineering. Send e-mail about this article to DE-Editors@digitaleng.news.
Follow DE