Delivering on Device Traceability
Can PLM deliver the traceability needed by medical device providers?

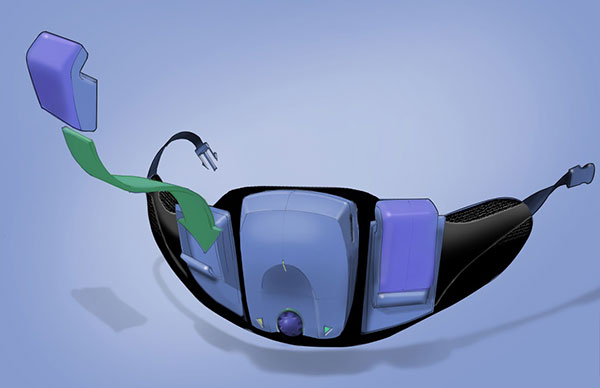
Wearables like the orthotic device shown here—which aims to provide the end user a better range of motion—require precise anatomical matching that typically calls for a one-off build. Image courtesy of BlackHägen Design.
Latest News
September 18, 2023
Today’s medical device providers must overcome various regulatory and market challenges. These range from government regulation and growing demand for customization to the increasing call for wireless and wearable devices. While each obstacle has its own unique characteristics, they all seem to share the need for a common element: comprehensive traceability (Fig. 1).
To cultivate the level of traceability that the medical device sector requires, companies have begun to turn to product lifecycle management (PLM) systems, taking advantage of their ability to manage not only their product data and specifications as design outputs but also the records that establish traceability throughout the entire design history. PLM advocates contend that these platforms will speed up the capture and retrieval of data, ensure data consistency across the organization and reduce the risk of human error.
As with so many of the digital tools in the spotlight today, however, users must distinguish between promised functionality and deliverable performance.
Thus, the fundamental question confronting medical device providers becomes: Can today’s PLM systems meet the current raft of challenges, as well as those emerging just over the horizon?
Furthermore, will PLM technology’s evolutionary path ultimately position the platforms to deliver the resolution required to validate medical device designs and processes?
Regulatory Compliance and Burden of Proof
Perhaps the best medical device application that can test PLM’s ability to provide the necessary traceability is regulatory compliance.
A key requirement for regulatory compliance is design control. This demands that medical device developers amass large amounts of evidence and data to demonstrate to auditors that their systems, processes and products comply with stringent governmental regulations and are eligible to be placed on the market. These challenges play directly to PLM’s strengths.
“To meet design control requirements, a comprehensive digitalization program, with a strong PLM platform, enables customers to orchestrate, capture records and later report exactly how their product was developed in the context of a product development plan,” says James B. Thompson, senior director of the medical device and pharmaceutical industries at Siemens Digital Industries Software.
Adding to this complexity is the dynamic regulatory landscape in which medical device companies operate.
“Governing regulations differ by product, geographic location and lifecycle stage,” says Purnima Krishnan, senior manager at Kalypso, a Rockwell Automation business. “To make matters worse, the regulations and standards constantly change. To meet these challenges, PLM platforms must support these regulations by native features and functionalities, and be constantly and rapidly upgraded to accommodate changes.”
Thus, medical devices companies shopping for a PLM platform should not only look for the agility to handle diverse data types but also the ability to support various applications.
To this end, PLM platforms should incorporate applications for project plans management, risk and requirements management, multidisciplinary design data and specification management, and verification and validation test planning and execution.
Medical device companies also need the PLM system to include quality management system functionality.
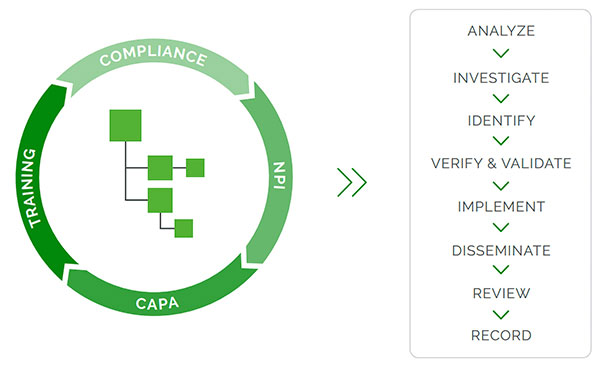
“Quality processes regulated in 21 CFR Part 820, like CAPA, are often linked to engineering processes—like engineering change orders (ECOs) or document change orders (DCOs)—and this is best done in a product-centric QMS system that manages quality and engineering processes together (Fig. 2),” says Ann McGuire, director of product marketing at Arena, a PTC business.
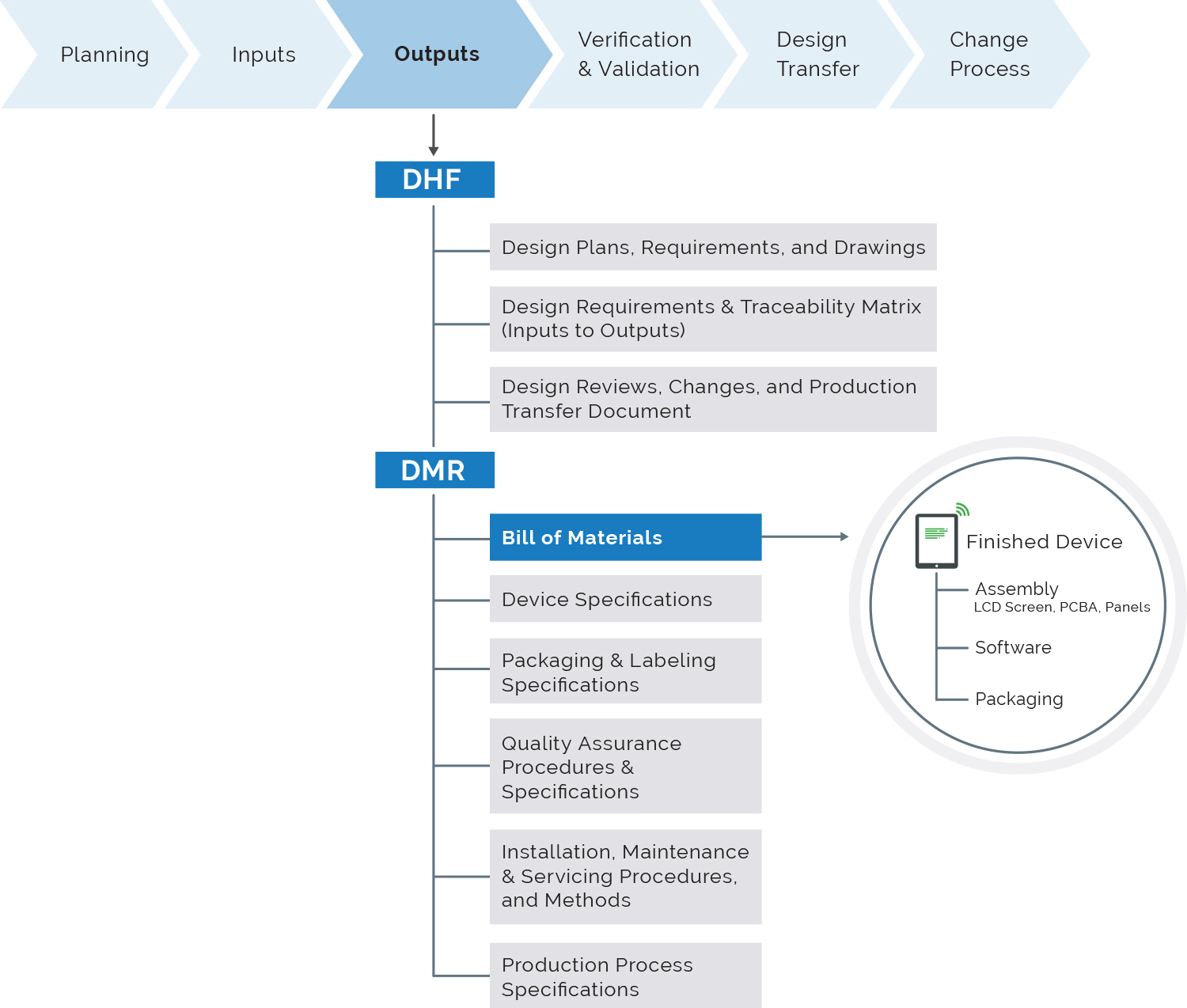
Importantly, the PLM platform should include a design history file (DHF) application.
“The DHF is vital for overall traceability and creation of configured views of the design history data and documents for re-use in design transfer to manufacturing as a device master record (DMR), and for content management of regulatory submissions for market clearance and periodic updates,” says Siemens’ Thompson.
PLM’s value, however, extends beyond the regulatory approval process. In addition to supporting an initial product development project, it is also important in tracking all product changes through its production and full evolutionary lifecycle to ensure continued safety and compliance. In addition, PLM enables companies to self-audit as well as support audits mandated by regulators.
Upon reviewing the chief challenges, it becomes clear that ensuring regulatory compliance requires robust traceability and documentation throughout the product lifecycle (Fig. 3). PLM systems have begun to meet these demands, but there is definitely room for improvement.
“PLM platforms need to support comprehensive traceability of design inputs, verification and validation activities, changes and regulatory compliance documentation,” says Krishnan. “While current PLM technology offers document management features, further advancements can be made to enhance traceability and documentation management capabilities.”
An Answer to Customization’s Complexity
Another trend challenging medical device companies is the growing demand for customized medical devices. This movement has led to a significant increase in the number of product configurations, variants and options within product lines, which in turn has exponentially magnified the complexity of regulatory compliance.
“Instead of a single, mass-produced device being designed and approved for production and distribution, it is the all-encompassing precision-medicine process to design, manufacture and adapt to patient-specific conditions,” says Thompson.
In the past, medical device developers have relied on grandfathered evidence and data to establish regulatory compliance. In doing so, they were able to introduce new products and devices quickly. Now, this practice is seen as a misuse of evidence, and it has forced PLM developers to take a new approach to up their game.
“Thankfully, with the speed and agility that PLM platforms now offer, the collection and analysis of new data and evidence is much quicker,” says Leigh Young, product manager at Aras. “The traceability that PLM platforms offer allows the reuse of historical designs, data and evidence, making it easier to document new product designs. While all the grandfathered data is still available and, in most cases, relevant, new data and evidence can be submitted for new product approvals alongside the old for a much more reliable and less risky data set.”
While the developers of the current generation of PLM systems have made advances, laying the groundwork required to meet new market demands, gaps in functionality remain.
“Current PLM technology provides variant-management capabilities, but there is room for improvement in terms of providing more flexible and efficient configuration management features to handle the increasing complexity,” says Krishnan.
Physical and Digital Merge in Wearables
With wearable medical devices, complexity comes in two flavors: the physical and the digital. Both add unique layers of complexity to the development process, making design control difficult to achieve (Fig. 4).
For example, wearable devices require an anatomical interface. This can take the form of a single-use component (e.g., an adhesive patch or a cannula) or a limited-use product (e.g., conformable or shapeable pads).
The interface can also be designed to be modular by combining a consumer element (e.g., durable controller/power unit) with the anatomical interface.
To complicate things further, wearables that require precise anatomical matching typically require a one-off build, driving up the complexity and cost through customization.
A design team may consider custom or off-the-shelf electronics to provide control and monitoring functions. Though the latter can considerably reduce the device’s overall cost, the mix-and-match approach that combines dedicated medical devices with consumer products can significantly impede efforts to achieve design goals like interoperability, validation and safety compliance.
Increasing demand for sustainable designs also adds to the challenges of customizable design because they affect material choices and component availability.
To manage the level of complexity introduced in these ways and to achieve greater lifecycle visibility, development teams require robust traceability and documentation throughout the product lifecycle.
“PLM platforms need to support comprehensive traceability of design inputs, verification and validation activities, changes and regulatory compliance documentation,” says Krishnan.
Unfortunately, the issues introduced on the digital side only exacerbate complexity and reinforce the need for traceability.
Any wearable device is going to be a smart device of sorts, and as a result, must support electronics and sensors. This means that wearable medical devices require the resources required to interpret the data that’s coming from the sensors and to store it securely, as well as wirelessly transmit the data somewhere, all of which places more demands on the development process. This introduces software into the mix.
As a result of the increasing prominence of software in wearable medical devices, PLM developers have been forced to cultivate new functionality in their platforms.
For example, this movement requires the ability to much more closely manage and monitor application testing to ensure that resources are focused on high-risk or important areas of the software. Armed with these capabilities, a test manager can better organize, control, analyze and trace the testing process while managing testing resources throughout the product lifecycle.
PLM platforms also need robust interfaces that help them integrate with other systems, such as electronic health records, cloud platforms, analytics tools and cybersecurity solutions.
“While current PLM technology has made progress in this area, further advancements are needed to ensure smooth data exchange, standardization and interoperability with emerging technologies and industry standards,” says Krishnan.
Making Distance-Care Devices Work
Connected devices collect data about the use of the device in the context of a diagnostic or therapeutic procedure, and transfer information to the healthcare provider or the medical device manufacturer to interpret and analyze the data before determining how to respond to the new information, which in turn might require sending information back to the device.
Much like wearable medical devices, these complex devices bring additional requirements to the design process. These include adding a high level of consumerism to the design to accommodate patient lifestyles.
“Simple operation, extreme reliability and cybersecurity raise the bar for traditional clinical devices because user needs and capabilities vary widely with the patient-as-operator,” says Philip Remedios, CEO and director of design and development at BlackHägen Design. “These devices may require a relatively long lifecycle per single patient to reduce overall costs. Therefore, they require modular construction for ease of maintenance and service. The predictability of likely failure modes at the design stage may not be straightforward, and the PLM team must pay particular attention to the failure mode and effects analysis process.”
If the design team chooses a mix that includes consumer-grade components or commercial off-the-shelf electronic devices, revalidation and obsolescence management must be integrated into the development process.
Another similarity between connected and wearable medical devices can be found in the complexity and design countermeasures triggered by the prominent role of software in connected devices.
“To support connected-care medical devices, companies must include as part of their medical device a software infrastructure that enables the device to send and receive data to either healthcare provider IT systems, the medical device manufacturer, or possibly both,” says Thompson. “This additional software would typically run in the cloud or even possibly in a healthcare facility’s IT environment.”
The software infrastructure should also support a variety of software systems that have not usually been incorporated in traditional PLM systems. Application lifecycle management, cloud and mobile low-code development and embedded systems for operating systems all have a role to play in a connected-care device.
In addition, provision should be made for maintenance functions.
“This means providing for software or firmware updates over the air to keep devices up to date,” says Aras’ Young. “Additionally, the system must be able to retain a record of what devices have received the updates and what have not.”
All challenges mentioned here mirror the increasing complexity found in other medical device designs. Without the ability to provide fully integrated development, manufacturing and service delivery traceability across the product lifecycle and all applicable engineering domains, to verify and validate that the devices work correctly, medical device companies will not be able to realize the full potential of the new connected-care scenarios.
Time for PLM to Step Up
The main challenges confronting medical device providers are the increase in product complexity and the associated demand for more documentation.
In the era of big data, the success of PLM platforms will be determined by their ability to manage design inputs, changes to design inputs and links with design outputs, such as bills of material, approved vendor lists, drawings and test results.
“The technology provides a singular, all-access platform that allows the data across the entire organization to be connected and accessed easier, facilitates quick and easy collaboration, streamlines impact analysis and enables tracking and traceability of each unit,” says Young.
The traceability aspect is likely one of the most important here, especially in recall situations. This allows the organization to track the individual unit on each user and regulate and mitigate the situation.
Another major challenge—which is also linked to data management—is the implementation of adequate security and privacy.
“With the increasing connectivity of medical devices, ensuring cybersecurity and data privacy becomes critical,” says Krishnan. “PLM platforms need robust security features, such as encryption, access controls, authentication mechanisms and vulnerability management. While current PLM technology includes basic cybersecurity measures, there is a continuous need to enhance security features to keep pace with evolving threats and industry best practices.”
PLM platforms provide a good starting point for addressing these issues, but the dynamic nature of market demands, as well as the ever-evolving technology, seems to have PLM almost constantly playing catch-up.
More Arena Solutions Coverage
More PTC Coverage
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News